Copyright 2008
Ashley Davidoff MD
Definition
Pulmonary thromboembolism is a disease resulting from occlusion of one or more pulmonary arteries by bland thrombus, tumor thrombus, fat, amniotic fluid or foreign bodies. It is a leading cause of morbidity and mortality particularly among hospitalized patients. Most commonly the thrombi embolize from the deep venous system of the lower extremities but other sources include pelvic veins, upper extremity veins, right heart chambers and in situ thrombosis.
Overview
Pulmonary embolism is a leading cause of morbidity and mortality and occurs in many clinical situations. It is responsible for approximately 50 000 deaths annually and has a much higher incidence, possibly as much as 500 000 per year. While there are many sources for pulmonary emboli, more than 95% occur from the deep venous system of the lower extremities. Risk factors for include immobilization, surgery, trauma, congestive heart failure, oral contraceptives, malignancy and pregnancy. Emboli cause increased pulmonary artery pressure and ischemia of the affected pulmonary parenchyma. Occlusion of a major pulmonary artery can lead to sudden increase in pulmonary artery pressure, diminished cardiac output, right sided heart failure and death. Clinical outcomes range from resolution (60-80%), pulmonary hemorrhage or infarct (20%), shock (5%), pulmonary hypertension (2-3%) and death (5-10%). Clinical manifestations include sudden onset of dyspnea, pleuritic chest pain and hemoptysis. Physical exam findings include tachycardia or signs of right heart failure in cases of massive PE. Findings of DVT is an excellent indicator of PE.
Principles
Although we have understood the basic pathophysiology of a pulmonary embolus for many years, the morbidity and mortality associated with this disease remains unacceptably high.
It is estimated that upto 500,000 suffer from pulmonary emboli each year and that upto 50,000 of those will die.
Unfortunately, many of the embolic events are instantly fatal and it is often difficult to make the diagnosis of pulmonary embolus in those who survive the initial event. The clinical presentation of these patients is usually non-specific and the meaning of our diagnostic tests is frequently misunderstood.
22153e 61833
General
Theodore Roosevelt
26th President of the United States, 1901-1909. Lived 1858-1919.
Roosevelt had had a pulmonary embolus three weeks prior to death.
Death was ascribed to a pulmonary embolus. Before he went to bed, on the night of his death, there was an episode of breathlessness . A servant noticed at 4 a.m. that Roosevelt’s breathing was irregular, and “went at once to the bedside.”
Venous thromboembolism is any thromboembolic event occurring
within the venous system, including deep vein thrombosis and
pulmonary embolism. Deep vein thrombosis is a radiologically
confirmed partial or total thrombotic occlusion of the deep venous
system of the legs sufficient to produce symptoms of pain or
swelling. Proximal deep vein thrombosis affects the veins above
the knee (popliteal, superficial femoral, common femoral, and iliac
veins). Isolated calf vein thrombosis is confined to the deep veins
of the calf and does not affect the veins above the knee.
Pulmonary embolism is radiologically confirmed partial or total
thromboembolic occlusion of pulmonary arteries, sufficient to
cause symptoms of breathlessness, chest pain, or both. Postthrombotic
syndrome is oedema, ulceration, and impaired viability
of the subcutaneous tissues of the leg occurring after deep vein
thrombosis. Recurrence refers to symptomatic deterioration owing
to a further (radiologically confirmed) thrombosis, after a previously
confirmed thromboembolic event, where there had been an initial
partial or total symptomatic improvement. Extension refers to a
radiologically confirmed new, constant, symptomatic intraluminal
filling defect extending from an existing thrombosis

Statistics
Pulmonary embolism is a leading cause of morbidity and mortality and occurs in many clinical situations. It is responsible for approximately 50 000 deaths annually and has a much higher incidence, possibly as much as 500 000 per year.
A prospective Scandinavian study found an annual incidence of 1.6–1.8/1000 people
in the general population. One postmortem study estimated that 600 000 people develop pulmonary embolism each year in the USA, of whom 60 000 die as a result.
Historical Aspects
In the 1800s Virchow described the classic triad of causative factors for venous thrombosis, including a state of hypercoagulability.
1963 Henry Wagner first used radiolabeled albumin aggregates for imaging lung perfusion in normal persons and patients with pulmonary embolism.
Until the 1990s, there were not many diseases that were known to predispose to hypercoaguability
In the early 1990’s the landmark Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study was published, indicating guidelines for interpretation of ventilation-perfusion studies.
MDCT introduced in the early years of the new millenium is now the method of choice for pulmonary embolism.
Standard inpatient treatment of deep venous thrombosis with 5 to 7 days of Now that there is more frequent use of low-molecular-weight heparin since the late 1990’s outpatient treatment of this entity is becoming standard.
Thrombolysis percutaneous embolectomy,IVC filter – only last order?

54467
Classification
I26 Pulmonary embolism
I26.0 Pulmonary embolism with mention of acute cor pulmonale
I26.9 Pulmonary embolism without mention of acute cor pulmonale
Fat Embolism
Tumor Embolism
Septic Embolism
Amniotic Fluid Embolism
Chronic Thrombotic Large Vessel Pulmonary Artery Obstruction
Chronic Small Vessel Pulmonary Thrombosis
Pulmonary Thromboembolism
 32190c
32190c
Etiology and Predisposing Factors
Risk factors for PE are related to deep venous thrombosis as lower extremity DVT are the source of over 95%.
Immobilization (prolonged bed rest, long airplane flights)
Malignancy
Surgery
Severe trauma (multiple fractures or burns)
Congestive heart failure
Oral contraceptives
Pregnancy
Stasis: DVT, Immobilization ( formed 2ry to cast, long-distance airplane travel, taxi/bus driver, etc.), Polycythemia, SC, Burns, Pregnancy, Obesity,
Cardiac: Arrhythmia, CHF, Cardiomyopathy, Indwelling Central Line.
Hypercoagulability: BCP (estrogen based), Malignancy, homocystine, protein C or S
Trauma: Postoperative state, Leg trauma,
Prior PE or DVT, protein C or S, LES (antiphospholipid).
Fat embolism (liposuction or bone fracture).
Risk factors for deep vein thrombosis include immobility, surgery
(particularly orthopaedic), malignancy, smoking, pregnancy, older
age, and inherited or acquired prothrombotic clotting disorder.
The oral contraceptive pill is associated with increased risk of death
due to venous thromboembolism (ARI with any combined oral
contraception: 1–3/million women a year). The principal cause of
pulmonary embolism is a deep vein thrombosis.
Geographical predisposition
This is a univerasal disease and has no geographic bounds.
Population Group
There are certain conditions including air travel, females on the OCP, as well as older patients who are not moving and are bed or chair bound, that predispose patient groups to the disease.
Genetics
There are a number of genetic aberrations that result in a hyprercoaguable state
factor V Leiden mutation is the most common inherited cause of hypercoaguability
Others include
hyperhomocysteinemia
Prothrombin G-A20210 gene variant – protein C deficiency
protein S deficiency
antithrombin III deficiency
Sex
Sex based risk factors include:
Preganancy
Oral contraceptives and estrogen-containing medications
Age
The risk for developing pulmonary embolus increases with age.
Dietary
No specific dietary factor has been identified.
There is an increased risk, however, with obesity.
Associated Diseases
Pulmonary embolus is associated with:
Stroke
Heart Disease
Peripheral Vascular disease
Deep Vein thrombosis
Diabetes
Fractures – (mainly lower extremity)
Malignancy
Pathogenesis
Since over 95% of all PE arise from thrombi within the large deep veins of the lower legs, the discussion of the pathogenesis will focus on thrombogenesis.
The factors that predispose to thrombosis are endothelial injury, stasis or turbulence of blood flow and hypercoagulability.
Stasis plays the dominant role in thrombogenesis in the venous side of the circulatory system because of low flow velocity. This is in contradistinction to the arterial side where endothelial injury is likely the dominant factor. Most often, venous side thrombosis starts behind the valve cusps in the deep venous system of the lower extremities.
Hypercoagulability is an alteration in the clotting mechanism predisposing to thrombosis.
Endothelial injury is an important factor in thrombogenesis. Potential causes include hypertension, bacterial toxins or endotoxins, toxins from cigarette smoking, homocystinuria and hypercholesterolemia. Endothelial damage exposes the subendothelial collagen which acts as a focus of platelet aggregation.
Complications
The clinical outcomes of pulmonary embolism are resolution (60-80%), pulmonary hemorrhage or infarct (20%), shock (5%), pulmonary hypertension (2-3%) and death (5-10%).
Most PE are small and therefore silent
The annual recurrence rate of symptomatic calf vein thrombosis in
people without recent surgery is over 25%. Proximal extension
develops in 40–50% of people with symptomatic calf vein thrombosis.
Proximal deep vein thrombosis may cause fatal or non-fatal
pulmonary embolism, recurrent venous thrombosis, and the postthrombotic
syndrome. One case series (462 people) published in
1946 found 5.8% mortality from pulmonary emboli in people in a
maternity hospital with untreated deep vein thrombosis. One
non-systematic review of observational studies found that, in
people after recent surgery who have an asymptomatic deep calf
vein thrombosis, the rate of fatal pulmonary embolism was
13–15%.10 The incidence of other complications without treatment
is not known. The risk of recurrent venous thrombosis and complications
is increased by thrombotic risk factors.
Natural History
The clinical outcomes of pulmonary embolism are resolution (60-80%), pulmonary hemorrhage or infarct (20%), shock (5%), pulmonary hypertension (2-3%) and death (5-10%).
Most PE are small and therefore silent
The annual recurrence rate of symptomatic calf vein thrombosis in
people without recent surgery is over 25%. Proximal extension
develops in 40–50% of people with symptomatic calf vein thrombosis.
Proximal deep vein thrombosis may cause fatal or non-fatal
pulmonary embolism, recurrent venous thrombosis, and the postthrombotic
syndrome. One case series (462 people) published in
1946 found 5.8% mortality from pulmonary emboli in people in a
maternity hospital with untreated deep vein thrombosis. One
non-systematic review of observational studies found that, in
people after recent surgery who have an asymptomatic deep calf
vein thrombosis, the rate of fatal pulmonary embolism was
13–15%.10 The incidence of other complications without treatment
is not known. The risk of recurrent venous thrombosis and complications
is increased by thrombotic risk factors.
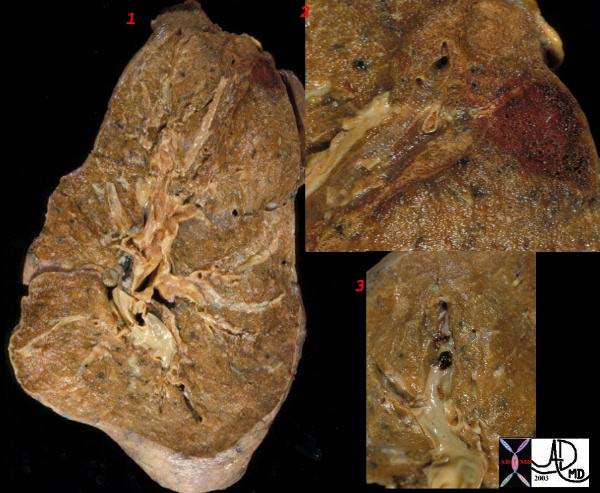
Gross Pathology
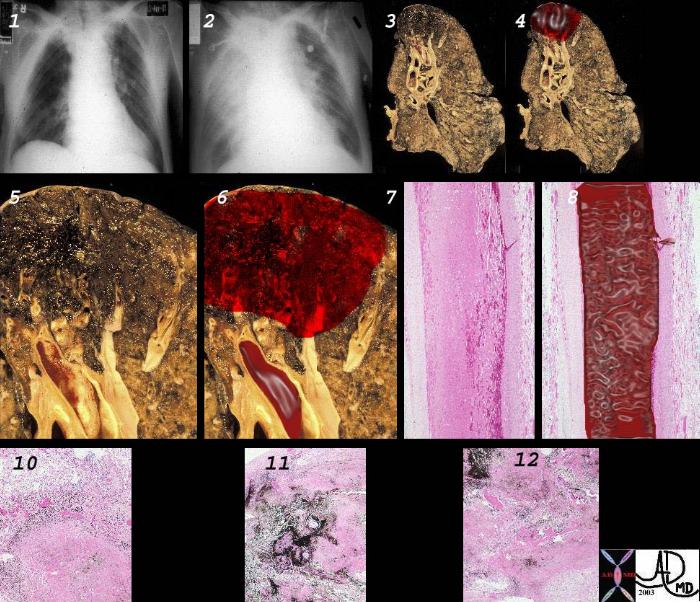
The gross pathologic findings of pulmonary embolism depends on the size of the embolus.
Large emboli that occlude the main pulmonary artery or its main branches can cause sudden death from hypoxia or acute right sided heart failure (acute cor pulmonale) that no changes in the lungs is seen.
Smaller emboli can become lodged in medium or small sized pulmonary arteries. When this occurs, ischemia of the affected lung parenchyma, if mild, can cause alveloar collapse and atelectasis from decreased surfactant production. If the ischemia is more severe, damage to the endothelial cells of affected alveoli results in pulmonary hemorrhage. If the there is compromised cardiac output, pulmonary infarction can occur. Pulmonary infarcts are commonly multiple and peripheral, typically occuring in the lower lobes. Pulmonary infarcts are wedge or triangular shaped with the base abutting the pleural and the point directed centrally.

32190c

32328c2
Diagnosis
Clinical
a) very inaccurate (about50%)
b) frequent historical findings:
pain
swelling
erythema
frequent physical findings:
Homan’s sign (in 20% patients with DVT & 30% without DVT)
palpable cord (in 30% patients with DVT & 30% of patients without DVT)
The patient may present in many ways.
Dyspnea, Pleuritic Chest pain, (may be present 3-4 days prior to diagnosis)
Tachypnea
Cough
Anxiety
Rales (50%)
Tachycardia (40 – 50%)
Low grade fever (40%)
DVT in about 30% of cases
Other presetations:
Sudden onset
One or multiple symptoms
Cough — may be blood-tinged
Shortness of breath
Chest pain may be worse with breathing or coughing
Pain may be on one side and may worsen with bending forward.
Anxiety and restlessness
Person may faint (syncope)
Sweating
Wheezing
Rapid shallow breaths
Cyanosis
Hypoxia
Rapid, pounding, or Racing Heart rate
Edema in the legs
Pain in the back of the legs
Joint pain
Dizziness
Pain in the pelvic areas
Sometimes no symptoms appear
Sudden death may occur
Lab
As a result of pulmonary embolism, blood gases may be altered.
A-a gradient = [(713) x (FiO2)] – [PaCO2 x 1.25] – PaO2.
Normal is 10 to 15 mmHg. The A-a gradient must be adjusted to age if pt is > 65 years. After the age of 60-65, PaO2 drops by about 1mmHg for every year.
Fibrin degradation products- Positive D-dimers 30-40% specific. Negative D-dimers are 90% sensitive in excluding PE and posite DVT/PE were witnessed in presence of negative D-dimers. Both depend on the methods (ELISA vs. latex, whole blood vs. plasma) used by hospital lab.
Check for protein C+ S deficiency
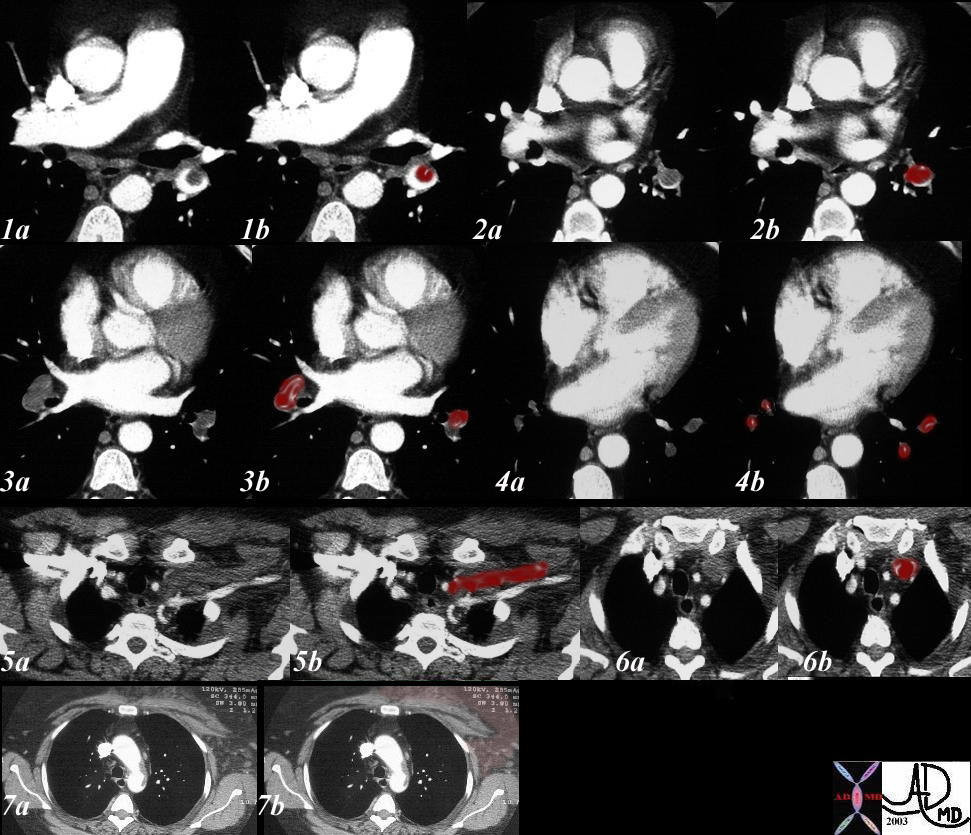
Imaging
V/Q scan. High probability V/Q is essentially diagnostic, and medium probability V/Q is diagnostic if there are other S & S that could create a high clinical suspicion of PE (O2 SAT <95%, DVT, SOB, >A-a gradient, Tachycardia, Tachypnea). If medium probability V/Q is not accompanied by S&S but pt has risk factors, Pulmonary Angio is the next step for dx. Patients with low probability V/Q have 10% chance of having PE, and patients with normal V/Q still have as much as a 5% chance of having PE. Risk factors, S & S and proper F/U must be taken into account in these categories of patients.
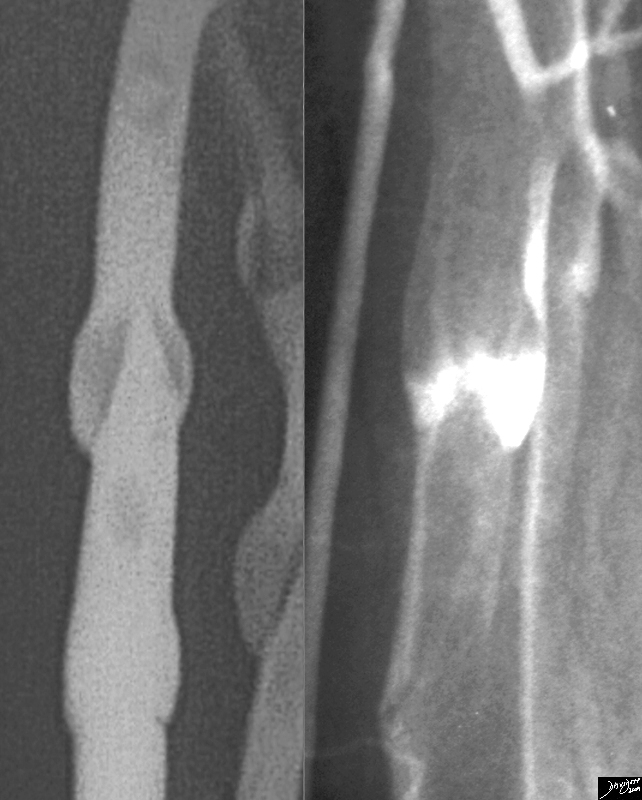
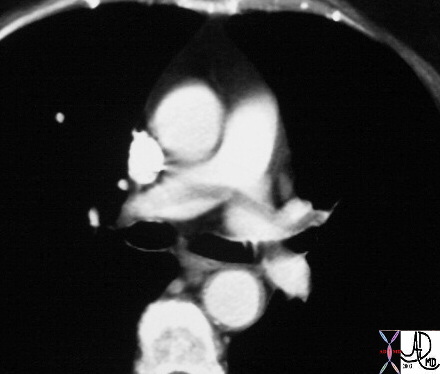
Spiral CT. This rules out only life threatening large PE but cannot r/o PE to small vascular bed. Negative CT should be regarded similarly to a “low” probability V/Q and pt should be treated on the basis of clinical exam and clinical suspicion.
ECHO. Patients unstable to leave the monitored bed would benefit from bedside ECHO. Clear signs of R ventricular failure may suffice for the dx of PE in proper setting, i.e. in pt with clinical evidence of PE. In addition it also helps to r/o other entities causing hypotension such as MI, Aortic Dissection, Tamponade.
Venous Doppler. When positive in the presence of pulmonary S & S and the presence of “low -to-medium probability” V/Q, Heparin treatment for PE is indicated.
Angiogram. Indicated when:
V/Q is low-mod probability and DVT study is negative, yet pt has high clinical suspicion based on S&S and/or risk factors.
Patient is High risk for bleed if put empirically on anticoagulants.
32538c03
Treatment and Management
O2
Heparin dose is 5000 – 10,000 U bolus followed by 25,000 U in 250 D5W @ 10 – 15cc/hr (or 17U / kg / hr). Peak effect in 20-60 min and T1/2 0.5-2.5 hr.
Enoxaparin (Lovenox) is used more frequently nowadays. Administered SQ. No PT, PTT monitoring is needed. Peak effect occurs after 3-5 hrs. Is eliminated renally, and RF prolongs T1/2, yet dose adjustment is not needed. Usual T1/2 is 12 hrs. Can be given in pregnancy. If reversal is needed Protamine is given; and 1 mg Protamine reverses 1 mg of Enaxaparin. The dose for treatment of acute PE is 1.5 mg/kg qd or 1 mg/kg SQ bid x at least 5 days, with warfarin started on day 2 (while Lovenox is continued until PT is therapeutic for 5 consecutive days).
Thrombolytics used only when documented PE causes hemodynamic compromise.
Embolectomy.
Prognosis
The clinical outcomes of pulmonary embolism are resolution (60-80%), pulmonary hemorrhage or infarct (20%), shock (5%), pulmonary hypertension (2-3%) and death (5-10%).
Most PE are small and therefore silent
The annual recurrence rate of symptomatic calf vein thrombosis in
people without recent surgery is over 25%. Proximal extension
develops in 40–50% of people with symptomatic calf vein thrombosis.
Proximal deep vein thrombosis may cause fatal or non-fatal
pulmonary embolism, recurrent venous thrombosis, and the postthrombotic
syndrome. One case series (462 people) published in
1946 found 5.8% mortality from pulmonary emboli in people in a
maternity hospital with untreated deep vein thrombosis. One
non-systematic review of observational studies found that, in
people after recent surgery who have an asymptomatic deep calf
vein thrombosis, the rate of fatal pulmonary embolism was
13–15%.10 The incidence of other complications without treatment
is not known. The risk of recurrent venous thrombosis and complications
is increased by thrombotic risk factors.